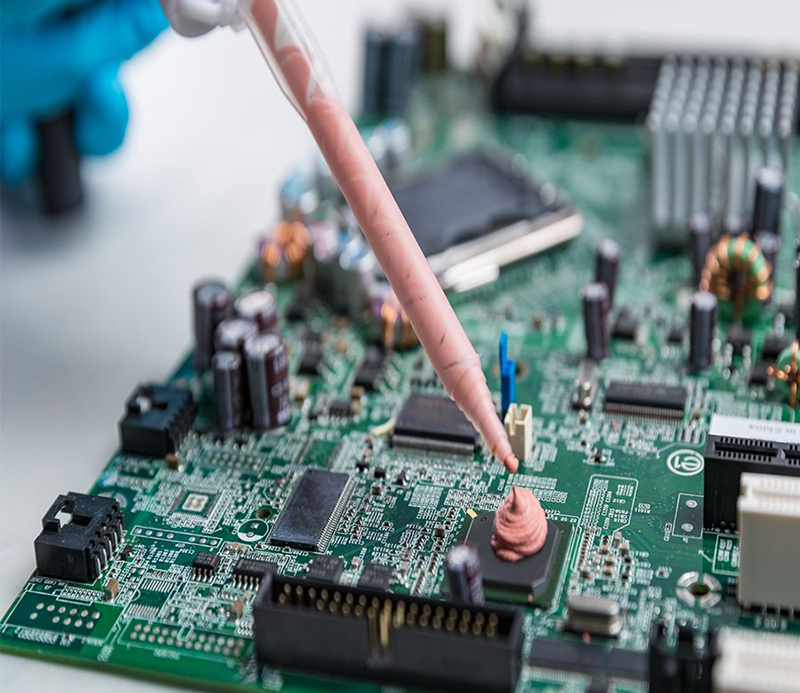
How to Extend the Surface Drying Time of Two-Component Thermal Conductive Gel?
Two-component thermal conductive gels are critical materials in thermal management for electronic devices due to their excellent thermal conductivity and high adhesion. In practical applications, extending the surface drying time of two-component thermal conductive gels is essential, especially for situations requiring long handling times or storage. Prolonging the surface drying time enhances application convenience, reduces waste, and ensures the gel maintains its optimal working condition during installation. NFION will delve into the factors affecting the surface drying time of two-component thermal conductive gels and explore effective strategies for prolonging it.

Basics of Two-Component Thermal Conductive Gel and Surface Drying Time
Two-component thermal conductive gels are composed of A and B components that, when mixed, undergo a chemical reaction to create a cured structure, achieving thermal conductivity and adhesion. Surface drying time refers to the period required for the gel to dry on the surface until it’s no longer tacky. This time determines the length of the working window and impacts its ease of use in specific environments.
Typically, the curing reaction speed of two-component thermal conductive gels is influenced by material composition, ambient temperature, and humidity. Under high-temperature and high-humidity conditions, the reaction accelerates, shortening the surface drying time. Therefore, controlling surface drying time is crucial for specific application needs.
Key Factors Affecting the Surface Drying Time of Two-Component Thermal Conductive Gel
1. Type and Concentration of Catalyst
The curing reaction of two-component thermal conductive gels generally depends on a catalyst, and both the type and concentration of the catalyst have a direct impact on the surface drying time. Higher concentrations lead to faster curing, reducing surface drying time, while lower catalyst concentrations extend the drying time. Adjusting the catalyst concentration is an effective way to control the drying time, but it’s essential to balance handling convenience and curing speed.
2. Ambient Temperature and Humidity
Temperature and humidity are critical external factors affecting surface drying time. Under high temperature and high humidity, the gel’s chemical reaction accelerates, shortening the drying time. In high-temperature environments, especially on production lines, controlling temperature and humidity is crucial. Lowering the ambient temperature and humidity can help extend the surface drying time.
3. Ratio of Components
The ratio of the A and B components directly impacts the reaction rate. Typically, an accurate ratio balances gel performance and curing time, while adjusting the ratio also affects the surface drying time. In scenarios requiring prolonged drying time, slightly reducing the proportion of component B can slow the reaction rate and extend the working window.
4. Type and Particle Size of Fillers
Fillers added to the conductive gel not only enhance thermal conductivity but also affect the curing process. Different types of fillers, such as alumina, boron nitride, or aluminum nitride, have variations in dispersion and particle size that cause minor changes in the gel’s curing reaction, influencing the surface drying time. Fine and evenly distributed filler particles can effectively slow down the curing process.

Effective Strategies for Prolonging Surface Drying Time
1. Reducing Catalyst Activity or Concentration
Selecting a less active catalyst or reducing the catalyst amount can effectively prolong the gel’s surface drying time. For example, in applications with low temperatures or humidity, reducing the catalyst concentration allows for a longer handling time without affecting the final thermal conductivity and adhesion.
2. Controlling Environmental Conditions
It is advisable to store and use thermal conductive gel in a low-temperature, low-humidity environment. Using air conditioning or dehumidifiers to reduce ambient temperature and humidity, especially on large production lines, can effectively extend the surface drying time and ensure gel performance stability.
3. Optimizing Gel Formulation
To meet specific application needs, inhibitors can be added to the formula, or filler ratios can be adjusted to slow the curing process. This adjustment can prolong the surface drying time without significantly affecting thermal performance. For example, adding titanate coupling agents or other low-reactivity inhibitors can effectively extend the gel's drying time.
4. Selecting the Appropriate A/B Component Ratio
To extend the surface drying time, slightly reducing the proportion of component B without affecting the final curing performance can provide a longer working window in actual operations, allowing operators more time to complete complex assembly steps.
Importance of Surface Drying Time Control in Practical Applications
Prolonging the surface drying time of two-component thermal conductive gels is highly beneficial for various application scenarios, specifically in:
● Enhancing Operational Flexibility: In complex electronic component assembly, extending the surface drying time gives operators sufficient time for installation and adjustment, preventing premature curing and reducing waste.
● Ensuring Device Performance: An optimal surface drying time ensures that the thermal conductive gel can fully cover the contact surface before curing, improving thermal performance and helping to extend the lifespan of electronic devices.
● Reducing Production Costs: As two-component thermal conductive gels are often high-cost materials, prolonging the surface drying time reduces unnecessary material waste, improves utilization, and lowers production costs.
Conclusion
The surface drying time of two-component thermal conductive gels plays a vital role in practical applications, especially in scenarios that require extended handling times or extensive application coverage. By adjusting catalyst concentration, controlling temperature and humidity, optimizing formulation, and modifying A/B component ratios, it is possible to effectively extend the surface drying time, enhancing application convenience and material efficiency. In actual operations, flexibly adjusting these parameters based on specific application needs can help achieve both thermal performance and economic benefits.



 CN >
CN >